https://www.unvaccinatedchildren.com/vaccine-free-quickstart-guide-for-parents/
Vaccine Free QuickStart Guide for Parents
By Larry Cook
The vaccine topic is very controversial and the amount of information about this topic on both sides (pro & anti) is staggering. I understand that it can be very difficult for you or any parent to sift through mountains of information and make heads or tails of it all. So, on this page I’m going to try and give you the fastest possible, most important points, with links to more information so you can do the in-depth research that you MUST DO in order to come to a conclusion that vaccines are neither safe, nor effective, and therefore, are not needed for your children, and were never needed for children. Let’s begin!
Vaccination & Natural Immunity
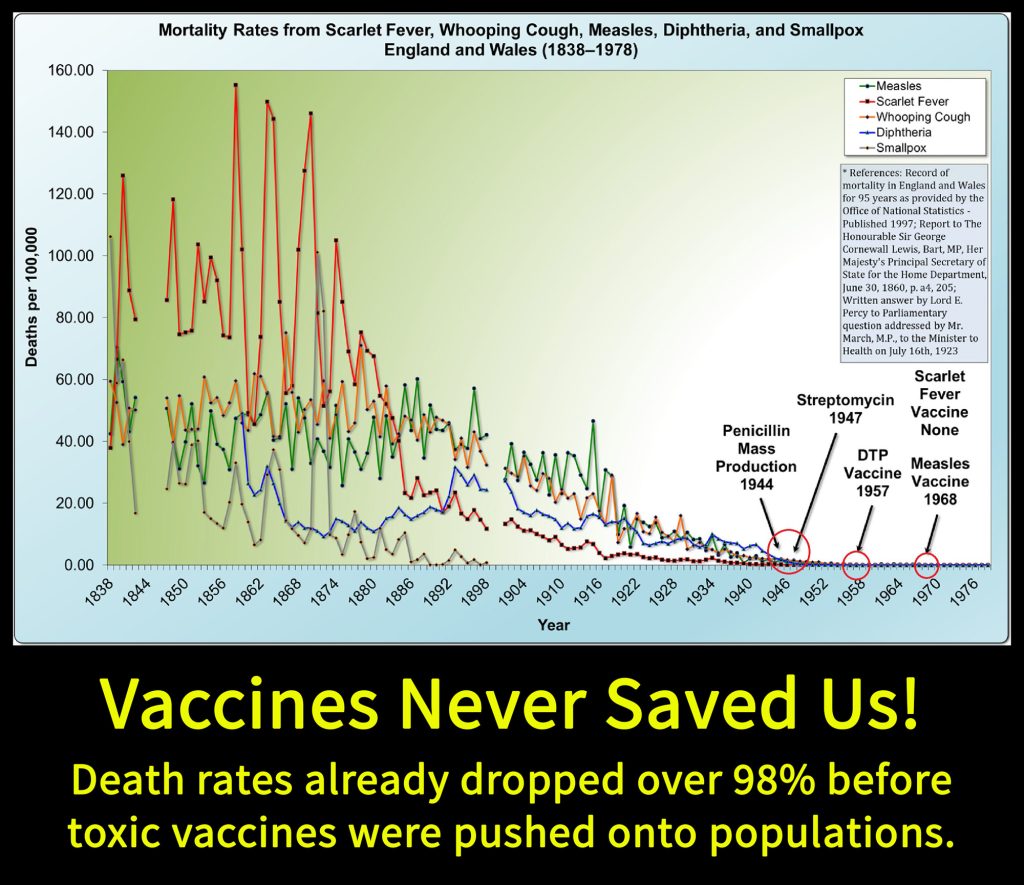
- Vaccines never saved us: DEATH from all infectious diseases dropped over 98% before most vaccines were introduced. Measles deaths dropped 99.96% before the vaccine was introduced. Death from Scarlet Fever also dropped in the same way, and there’s no vaccine for it.
- Unvaccinated children are healthier than vaccinated children according to numerous studies and the parents who have both (scroll down for more info).
- Vaccines destroy immune CAPACITY, which is why unvaccinated children recover from illnesses faster than vaccinated children.
- HEALTHY children do not die from infections – malnourished children do. Vaccines are not the solution – proper nutrition is. Vitamins A & C easily treat measles, for example.
- HOMEOPATHY was used in the 1800s and early 1900s to stop disease outbreaks, but was stopped in the early 1900s by the allopathic medical lobby to make way for toxic vaccines and drugs.
- High dose vitamin C – in the form of IVs, IMs and oral use – was used successfully to treat and cure a wide variety of diseases, including polio, since the 1950s, and is still used today by alternative doctors.
- Vaccines do not confer immunity – meaning, they do not work. When a child winds up with the symptoms the child was vaccinated for, the unvaccinated are blamed, instead of the faulty vaccination PROCESS.
- Healthy, well nourished children recover from childhood illnesses, and respond well to herbs, homeopathy, nutritional supplements and other safe and effective remedies – see book below.
- Vaccines SUBDUE immune response, but this is NOT immunity – infections just go deeper into the body.
- Vaccines aggressively overstimulate the immune system in order to create antibodies to injected antigens, but again, that is not immunity AND that overstimulation of the immune system never dies down entirely, giving rise to chronic health ailments.
- Children have lived on in spite of being poisoned by vaccines, NOT BECAUSE OF IT (extremely important).
- Vaccines cause autoimmune disorders, type 1 diabetes, cancer, asthma, allergies, eczema, seizures, autism, death, and dozens of other lifelong damaging ailments. See Vaccine Dangers for full overview.
- Vaccines ALWAYS cause some harm, and often cause a lot of harm. See my Vaccine Injury Treatment Guide if your child is vaccine injured.
- VACCINE EXEMPTIONS are available in most states in order for children to attend school or daycare without vaccines. Click either exemption information or California exemption information for details.
(See link for article and resources)
For more:
- https://madisonarealymesupportgroup.com/2015/06/19/a-word-on-vaccines/
- https://madisonarealymesupportgroup.com/2017/11/28/biological-mechanisms-of-vaccine-injury/
- https://madisonarealymesupportgroup.com/2018/04/09/a-tale-of-3-metals-the-fate-of-western-civilization-what-we-can-do-about-it/
- https://madisonarealymesupportgroup.com/2019/01/07/the-vaccine-debate-top-government-expert-states-vaccines-can-cause-autism-in-some-children/
- https://madisonarealymesupportgroup.com/2024/05/20/the-vaccine-war-a-multi-decade-attempt-to-poison-kill-and-disable/
- https://madisonarealymesupportgroup.com/2020/11/10/flu-vaccine-education/
- https://madisonarealymesupportgroup.com/2019/06/13/blast-from-the-past-cdc-vaccine-authors-destroy-evidence-of-vaccine-harm/
- https://madisonarealymesupportgroup.com/2026/02/03/simpsonwood-the-day-vaccine-evidence-disappeared/
- https://madisonarealymesupportgroup.com/2021/05/18/notices-of-liability-for-covid-19-vaccine-harms-and-deaths-served-on-all-members-of-the-european-parliament-evidence-based-position-paper-to-ensure-ethical-conduct/



