Review: Borrelia Miyamotoi
https://danielcameronmd.com/review-borrelia-miyamotoi/
REVIEW: BORRELIA MIYAMOTOI

Borrelia miyamotoi is an emerging tick-borne illness that is transmitted by the deer tick. The most common symptoms of a B. miyamotoi infection include fever, fatigue, headache, chills, myalgia, arthralgia, and nausea.
In their article, “Human Borrelia miyamotoi Infection in North America,” Burde and colleagues discuss the frequency and location of infection in ticks and people, clinical presentation and complications, diagnosis, treatment, and prevention.
Prevalence of B. miyamotoi
B. miyamotoi-infected ticks have been reported throughout the northeastern, northern Midwestern, and western United States. They’ve also been detected in all Canadian provinces except Newfoundland and Labrador.
The prevalence of Borrelia miyamotoi infections is difficult to determine, since the illness is not nationally reportable in the U.S. but reportable in only a few states including Connecticut, Maine, Massachusetts, Minnesota, New Jersey, Vermont, and Wisconsin. And, confirmation of the diagnosis depends upon laboratory testing, which is not always available.
Furthermore, diagnosis can be challenging. “The discrepancy between diagnosed and undiagnosed infection is probably even greater for B. miyamotoi, a tick-borne disease that lacks an easily identifiable clinical marker, such as the erythema migrans rash, and is less well known by health care workers and the general public,” the authors write.
Transmission
B. miyamotoi can be transmitted to humans through the bite of an infected black-legged (deer) tick. Several studies have found that it may be transmitted through blood transfusions, as well.
The B. miyamotoi pathogen can be transmitted from an infected female tick to her eggs, which may result in some larval ticks harboring the infection and transmitting it to a host. “Other larvae become infected after taking a blood meal on an infected mouse reservoir host, molt to the nymphal stage, and then transmit infection to another mouse or human,” they write.
Symptoms & Treatment
B. miyamotoi symptoms can be non-specific and an individual may appear to have a viral-like illness with fever, chills, headache, myalgia, fatigue, arthralgia, and gastrointestinal complaints, according to the authors.
“The most striking clinical feature of B. miyamotoi is relapsing fever with an initial febrile episode followed by a period of wellness and then one or more additional febrile episodes,” the authors write.
Some studies have found that the “average time between relapses was 9 days with a range of 2 days to 2 weeks.”
However, not all individuals develop relapsing fever. “In the largest case series of B. miyamotoi cases in the US, only 2 of 51 cases (4%) developed relapsing fever.”
READ: Don’t Rely on Relapsing Fever to Diagnose B. miyamotoi
Treatment of B. miyamotoi disease typically involves using the same antibiotics to treat Lyme disease: doxycycline, tetracycline, erythromycin, penicillin, and ceftriaxone. However, there have been no trials to evaluate the effectiveness of these treatments.
Co-infections worsen disease
Co-infections can worsen the illness. There have been reported cases of B. miyamotoi co-infection with B. burgdorferi and/or Babesia microti.
“Previous studies have found that coinfection of B. burgdorferi with either Babesia microti or with Anaplasma phagocytophilum are often associated with more severe disease compared with that caused by B. burgdorferi infection alone,” the authors write.
Testing for the infection can include blood smear, polymerase chain reaction (PCR), and/or antibody detection.
Authors’ Conclude:
“The possibility of B. miyamotoi infection should be considered in any patient with a febrile illness who resides in or has recently traveled to a region where Lyme disease is endemic, especially during the late spring, summer, or early fall.”
Related Articles:
B. miyamotoi can be transmitted from mother ticks to offspring
Could B. miyamotoi infections explain persistent Lyme disease symptoms?
References:
- Burde J, Bloch EM, Kelly JR, Krause PJ. Human Borrelia miyamotoi Infection in North America. Pathogens. 2023 Apr 3;12(4):553. doi: 10.3390/pathogens12040553. PMID: 37111439; PMCID: PMC10145171.
_______________
For more:
- https://madisonarealymesupportgroup.com/2020/11/18/what-you-need-to-know-about-borrelia-miyamotoi/
- https://madisonarealymesupportgroup.com/2022/03/29/borrelia-miyamotoi-found-in-3-5-of-new-england-blood-samples-tens-of-thousands-possibly-infected/
- https://madisonarealymesupportgroup.com/2020/12/14/how-many-negative-lyme-tests-are-due-to-b-miyamotoi/
- https://madisonarealymesupportgroup.com/2020/12/31/lyme-disease-are-we-looking-for-the-wrong-culprit/
- https://madisonarealymesupportgroup.com/2020/11/05/your-lyme-disease-test-results-are-negative-but-your-symptoms-say-otherwise/ Excerpts:
- Among positive ticks, 60% were positive for B. miyamotoi.
- Testing on over 2,000 humans (mainly late stage/chronic patients) showed 30% negative results and 70% positive, among which over 60% indicated the presence of specific Borrelia miyamotoi phages. https://madisonarealymesupportgroup.com/2020/11/30/neglected-infections-gastrointestinal-issues-in-patients-with-late-vector-borne-infections/
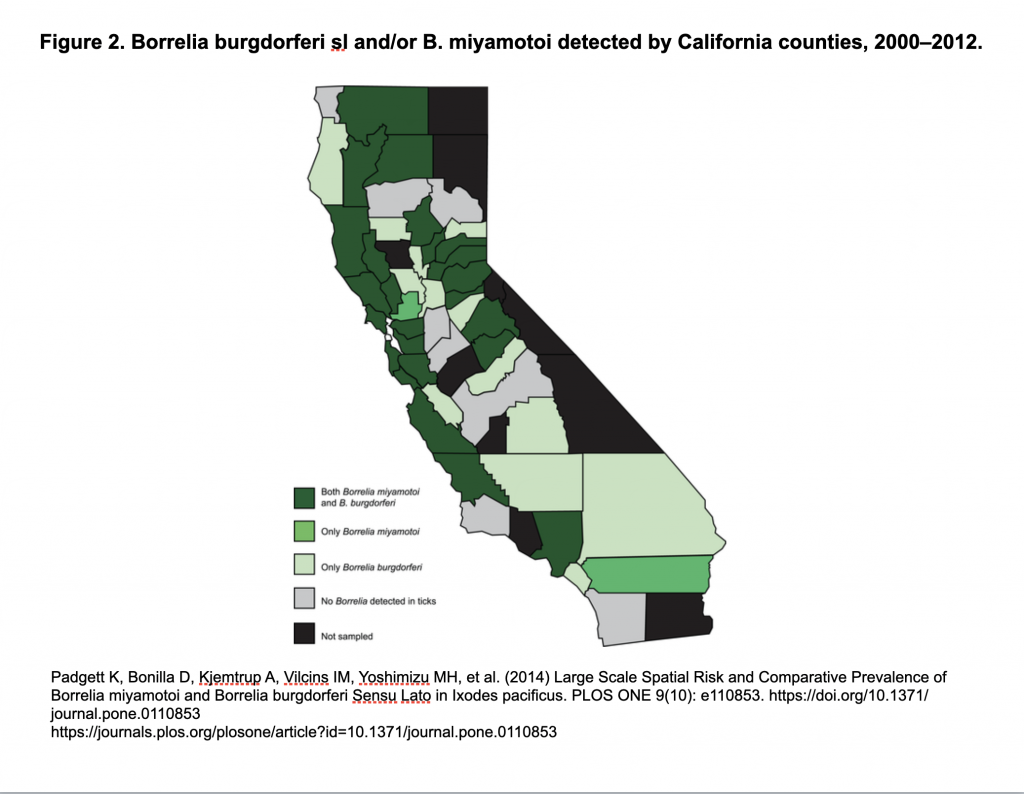
Also, Borrelia miyamotoi has been in California ticks for a long time:
https://madisonarealymesupportgroup.com/2018/02/15/b-miyamotoi-in-ca-ticks-for-a-long-time/
The following case shows how you can become infected while traveling: https://madisonarealymesupportgroup.com/2020/10/24/a-case-of-borrelia-miyamotoi/