LSU Obtains Grant to Synthesize Affordable Nootkatone
https://www.lsu.edu/eng/news/2024/03/che-nsf-pfi-grant-lyme-disease.php
Chemical Engineering, Biological Sciences Faculty Receive Largest NSF PFI Grant Ever Awarded to LSU
Nootkatone Studies Could Lead to Prevention of Lyme Disease
March 25, 2024
BATON ROUGE, LA – Thanks to a $550,000 National Science Foundation Partnership for Innovation grant—the largest NSF PFI grant ever awarded to LSU—LSU Chemical Engineering (ChE) Professor Kerry Dooley, LSU ChE Department Chair and Professor Mike Benton, and LSU Department of Biological Sciences (Biol. Sci.) Professor Roger Laine will continue their work on a project that could bring affordable and effective insect repellent to the masses, possibly decreasing the number of Lyme disease, malaria, and West Nile virus cases around the world.
The project involves the use of nootkatone, an FDA-approved organic compound found in grapefruit skin and Alaska yellow cedar trees that is a natural deterrent for many insects, including the deer tick responsible for Lyme disease. The LSU researchers propose decreasing the cost of the nootkatone synthesis, making any products made with the compound affordable to the general public.
“The family of compounds that make up nootkatone is already proven to be both safer and more effective than existing commercial repellents,” principal investigator Dooley said. “However, it’s now too expensive for consumer insect repellents. We plan to greatly streamline, optimize, and reduce the costs associated with the synthesis.”
According to Laine, there have been few insect repellents on the market since DEET, which is found in most insect repellent sprays and creams currently available. However, a mosquito test showed that nootkatone at 5% in rubbing alcohol was superior to DEET, which usually needs to be administered at greater than 20% concentration, even six hours after application.
Years ago, Laine discovered the efficacy of nootkatone as an insect repellent while collaborating with retired LSU AgCenter Entomologist Gregg Henderson in Laine’s lab. They found that nootkatone repelled insects like mosquitos, gnats, wood ticks, fleas, termites, lice, and fire ants because the insects weren’t eating the vetivone grass that, unbeknownst to them, contained nootkatone. Former LSU Biol. Sci. Senior Research Associate Betty Zhu tested 15 other compounds that resembled the structure of the vetivone and discovered that nootkatone was the best repellent compound. Nootkatone had already been approved by the FDA at the time, with the CDC later discovering that it also repels deer ticks.
Though nootkatone was found to be the best repellent, the problem was the cost to buy it in pure form.
“Nootkatone costs $2,500/kg, which is too costly for insect repellent,” Laine said. “It should be $200-$300/kg, then you can add it to lotions and sunscreens.”
Dooley discovered that one important way to save on the cost would be to modify a step in the eight-step synthesis of nootkatone.
“I did a cost analysis of the synthesis process, and 70% of the cost is concentrated in the fourth step of the eight-step process,” Dooley said. “I decided this step in particular could be significantly reduced in cost.”
The eight-step synthesis was created in just two years by former LSU Chemistry Graduate Student Anne Sauer, who was working under retired professor William Crowe as a collaboration with Laine and Henderson. To simplify two oxidation steps in the eight-step synthesis, which is patented by LSU, Laine subsequently obtained a Board of Regents seed grant and hired synthetic chemist Xuefeng Gao to successfully modify the synthesis using ozone, now covered by new U.S. and Japanese LSU patents authored by Laine.
In the fourth step, the original paper and patent uses potassium hydride and 18-Crown-6 ether, along with tetrahydrofuran, as a solvent. Dooley read up on how people were trying to execute this step without using these expensive components and thought he and Benton should come up with a catalyst and solvent that could significantly reduce the cost of this step.
“It’s incredibly complicated and it takes a long, sustained effort to go from making a few grams of something to making kilograms or kilotons,” Dooley said. “There’s a lot of work on optimizing separations, minimizing the solvent use, getting certain impurities down, and getting yields slightly up. These things take a lot of time and effort.”
The LSU research team hopes to sell their synthesis process to a manufacturing company, who would then be able to mass produce affordable nootkatone products that could save people’s lives by preventing bites from infectious insects.
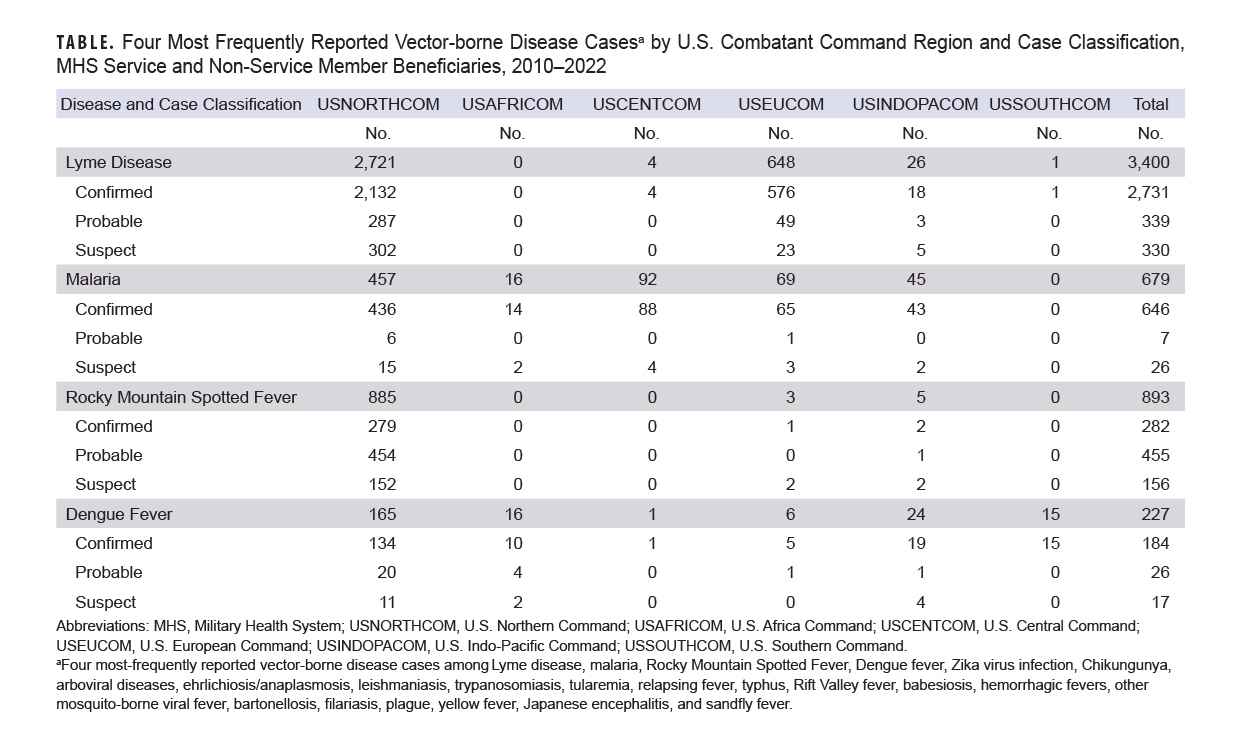
A 2024 CDC report states that there were 62,551 Lyme disease cases in 2022. Recent estimates using new data collection methods suggest approximately 476,000 people may be diagnosed with Lyme disease each year in the U.S.
“The deer tick is spreading throughout the U.S.,” Dooley said. “It’s not just prevalent in the Northeast.”
In other words, insects are going nowhere. A 2023 CDC report states there were 2,406 cases of West Nile virus across 43 states with the number of cases expected to increase in 2024. Per the World Health Organization, there were a reported 249 million cases of malaria worldwide last year.
“Sixty million people die of malaria each year,” Laine said. “It’s possible that if this eight-step synthesis process could produce nootkatone products that get to poorer countries, then WHO could possibly fund it. Mosquito nets could be covered with it, or they could have cloth ankle bands with nootkatone so ticks can’t crawl up your leg. The Department of Defense is also interested in ways to protect military personnel against tick-borne diseases. There are a lot of marketing niches with this.”
Contact: Libby Haydel
Communications Manager
225-578-4840
ehaydel1@lsu.edu
For more:
- https://madisonarealymesupportgroup.com/2017/01/10/nootkatone/
- https://madisonarealymesupportgroup.com/2018/04/12/nootkatone-against-ticks/
- https://madisonarealymesupportgroup.com/2020/08/11/nootkatone-registered-by-epa-insect-repellent-products-could-be-available-by-2022/
- https://madisonarealymesupportgroup.com/2019/04/12/tick-prevention-2019/
- https://madisonarealymesupportgroup.com/2019/07/18/frequent-prescribed-fires-can-reduce-risk-of-tick-borne-diseases/
- https://madisonarealymesupportgroup.com/2018/04/03/fire-good-news-for-tick-reduction/
- https://madisonarealymesupportgroup.com/2021/03/30/take-steps-now-to-protect-you-your-family-from-tick-bites/
- https://madisonarealymesupportgroup.com/2021/07/08/evaluating-effects-of-minimal-risk-natural-products-for-control-of-black-legged-ticks/