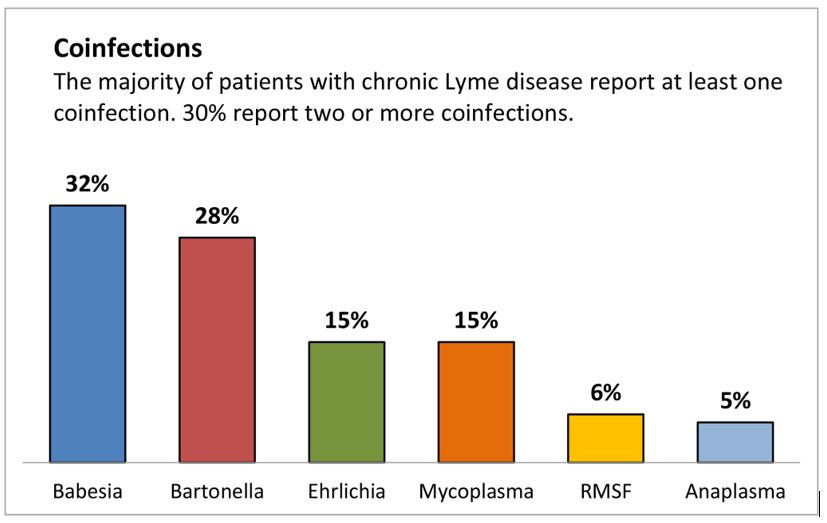
Landmark Study: Vaccination is the Dominant Risk Factor For Autism
UPDATE:
Go here to listen to Nicolas Hulscher and Dr. Andrew Wakefield discuss the landmark autism report.
https://www.thefocalpoints.com/p/breaking-landmark-report-finds-vaccination?
BREAKING — Landmark Report Finds Vaccination Is the Dominant Risk Factor for Autism Spectrum Disorder
McCullough Foundation’s authoritative analysis of more than 300 studies provides the most comprehensive synthesis to date on the possible causes of autism.
For decades, scientists have debated what drives the relentless rise in autism. Some have claimed it’s due to “increased screening” while others declare it’s anything but vaccines. Thousands of studies have explored genetic, environmental, and perinatal factors—but very few have ever examined vaccine and non-vaccine determinants together within a unified analytical framework.
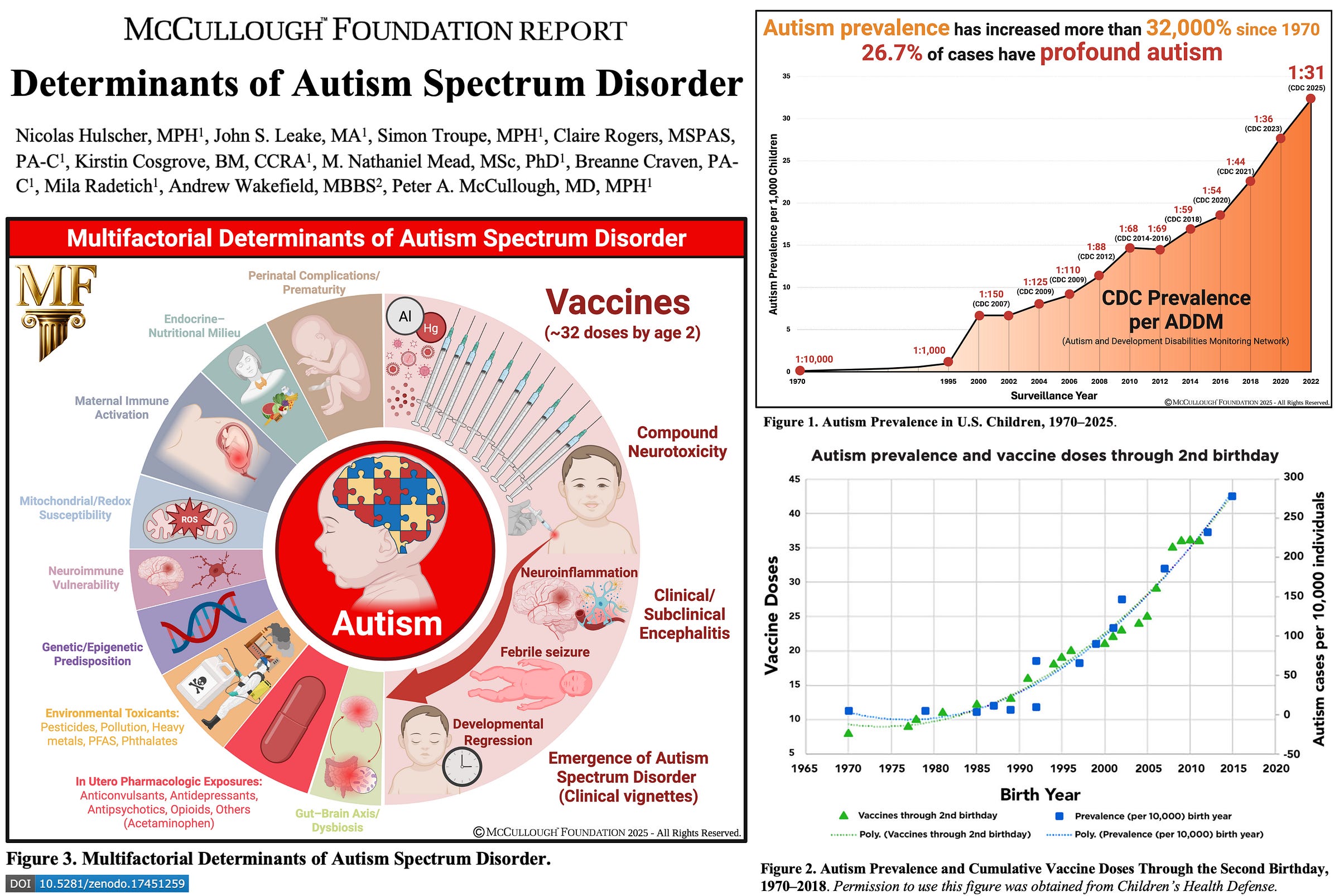
Now, the landmark McCullough Foundation Report titled, Determinants of Autism Spectrum Disorder, provides the most comprehensive synthesis on the possible causes of autism to-date. Thanks to the tireless work of Nicolas Hulscher, MPH, John S. Leake, MA, Simon Troupe, MPH, Claire Rogers, MSPAS, PA-C, Kirstin Cosgrove, BM, CCRA, M. Nathaniel Mead, MSc, PhD, Bre Craven, PA-C, Mila Radetich, Andrew Wakefield, MBBS, and Peter A. McCullough, MD, MPH — and support from the Bia-Echo Foundation — this historic effort was made possible.
Our report represents a major breakthrough through the iron grip of censorship imposed by the Bio-Pharmaceutical Complex on the issue of vaccination and autism. It also marks Dr. Andrew Wakefield’s first major return to the scientific literature in years—after enduring years of irrational attacks from the vaccine cartel.
By systematically integrating more than 300 studies across epidemiologic, clinical, mechanistic, and molecular domains, our team delivers the most extensive mapping yet of autism’s multifactorial origins and opens a new line of inquiry into how environmental and iatrogenic exposures intersect with genetic susceptibility.
By evaluating all known risk factors side by side, this analysis uniquely clarifies the relative contribution of vaccination compared to genetic and environmental domains. No prior review has attempted this integrative scope without excluding positive vaccine-association studies or unvaccinated controls—an essential step in determining whether vaccines truly play a role in autism risk, and if so, how significant that role is within the broader causal landscape.
Here’s what we found as described in the Abstract:
Introduction: Autism spectrum disorder (ASD) is now estimated to affect more than 1 in 31 children in the United States, with prevalence rising sharply over the past two decades and posing an increasing burden to families and public health systems. Most of the literature on ASD characterizes it as a complex neurodevelopmental condition shaped by multiple determinants, including genetic liability, immune dysregulation, perinatal stressors, and environmental toxicants. Since 1996, the possible role of childhood vaccination has also been discussed and debated. This review synthesizes the full range of evidence to clarify both vaccine-related and non-vaccine contributors to ASD risk.
Methods: We comprehensively examined epidemiologic, clinical, and mechanistic studies evaluating potential ASD risk factors, assessing outcomes, exposure quantification, strength and independence of associations, temporal relationships, internal and external validity, overall cohesiveness, and biological plausibility.
Results: We found potential determinants of new onset ASD before the age of 9 years old to include: older parents (>35 years mother, >40 years father), premature delivery before 37 weeks of gestation, common genetic variants, siblings with autism, maternal immune activation, in utero drug exposure, environmental toxicants, gut-brain axis alterations and combination routine childhood vaccination. These diverse genetic, environmental, and iatrogenic factors appear to intersect through shared pathways of immune dysregulation, mitochondrial dysfunction, and neuroinflammation, culminating in neurodevelopmental injury and regression in susceptible children. Of 136 studies examining childhood vaccines or their excipients, 29 found neutral risks or no association, while 107 inferred a possible link between immunization or vaccine components and ASD or other neurodevelopmental disorders (NDDs), based on findings spanning epidemiologic, clinical, mechanistic, neuropathologic, and case-report evidence of developmental regression. 12 studies comparing routinely immunized versus completely unvaccinated children or young adults consistently demonstrated superior overall health outcomes among the unvaccinated, including significantly lower risks of chronic medical problems and neuropsychiatric disorders such as ASD. The neutral association papers were undermined by absence of a genuinely unvaccinated control group—with partial or unverified immunization even among those classified as unvaccinated—alongside registry misclassification, ecological confounding, and averaged estimates that obscure effects within vulnerable subgroups. Only a few case–control studies verified vaccination through medical records or parent-held cards, and none performed independent clinical assessments of the children for ASD. In contrast, the positive association studies found both population signals (ecologic, cohort, case–control, dose–response, and temporal clustering) and mechanistic findings converging on biologic plausibility: antigen, preservative, and adjuvant (ethyl mercury and aluminum) induced mitochondrial and neuroimmune dysfunction, central nervous system injury, and resultant incipient phenotypic expression of ASD. Clustered vaccine dosing and earlier timing of exposure during critical neurodevelopmental windows appeared to increase the risk of ASD. These findings parallel strong, consistent increases in cumulative vaccine exposure during early childhood and the reported prevalence of autism across successive birth cohorts. To date, no study has evaluated the safety of the entire cumulative pediatric vaccine schedule for neurodevelopmental outcomes through age 9 or 18 years. Nearly all existing research has focused on a narrow subset of individual vaccines or components—primarily MMR, thimerosal-containing, or aluminum-adjuvanted products—meaning that only a small fraction of total childhood vaccine exposure has ever been assessed for associations with ASD or other NDDs.
Conclusion: The totality of evidence supports a multifactorial model of ASD in which genetic predisposition, neuroimmune biology, environmental toxicants, perinatal stressors, and iatrogenic exposures converge to produce the phenotype of a post-encephalitic state. Combination and early-timed routine childhood vaccination constitutes the most significant modifiable risk factor for ASD, supported by convergent mechanistic, clinical, and epidemiologic findings, and characterized by intensified use, the clustering of multiple doses during critical neurodevelopmental windows, and the lack of research on the cumulative safety of the full pediatric schedule. As ASD prevalence continues to rise at an unprecedented pace, clarifying the risks associated with cumulative vaccine dosing and timing remains an urgent public health priority.
- https://madisonarealymesupportgroup.com/2025/04/30/autism-guide/
- https://madisonarealymesupportgroup.com/2019/12/22/identification-evaluation-and-management-of-children-with-autism-spectrum-disorder/
- https://madisonarealymesupportgroup.com/2017/10/26/clinical-trial-shows-most-kids-with-autism-are-not-born-with-it/
- https://madisonarealymesupportgroup.com/2018/09/28/toxic-metal-pollution-linked-with-development-of-autism-spectrum-disorder/
- https://madisonarealymesupportgroup.com/2017/09/19/autism-aluminum-adjuvant-link-corroborated/
- https://madisonarealymesupportgroup.com/2016/12/08/mercury-and-autism/
- https://madisonarealymesupportgroup.com/2022/07/19/does-unrecognized-lyme-in-mothers-lead-to-autism-spectrum-disorder/
- https://madisonarealymesupportgroup.com/2020/08/09/what-lyme-autism-have-in-common-will-surprise-you/
- https://madisonarealymesupportgroup.com/2024/05/22/lyme-autism-dr-jodie-dashore/
- https://madisonarealymesupportgroup.com/2023/02/28/update-on-young-man-with-autism-bartonella-lyme/
- https://madisonarealymesupportgroup.com/2023/03/20/when-treating-bartonella-clears-symptoms-of-autism-what-next/