More Evidence Lyme Disease Can Persist After Treatment
https://www.lymedisease.org/lyme-can-persist/
Even more evidence that Lyme disease can persist after antibiotics
1/27/26
A review of the medical literature has found long-term infection in animal models and persistent infection despite antibiotic therapy in humans with ongoing symptoms of Lyme disease. The study was published in the open access journal Advances in Infectious Diseases.
Lyme disease is a tick-borne infection caused by Borrelia burgdorferi, a type of corkscrew-shaped bacteria known as a spirochete.
In 2021, the Centers for Disease Control and Prevention announced that Lyme disease is much more common than previously thought, with over 476,000 new cases diagnosed each year in the United States.
That makes Lyme disease seven times more common than hepatitis C virus infection, 15 times more common than HIV/AIDS and 49 times more common than tuberculosis in the United States.
The current study was conducted by nurse practitioner Melissa Fesler and internist Raphael Stricker from Union Square Medical Associates, a multispecialty medical practice in San Francisco, and Lorraine Johnson, chief executive of the patient support group LymeDisease.org.
Review identifies long-term infection in both people and animals
“Our findings address a major controversy over persistent symptoms in Lyme disease,” said Fesler, an author of the published study. “The results suggest that infection with the Lyme spirochete persists in some patients despite supposedly adequate antibiotic therapy.”
Previous studies have shown that the Lyme spirochete could survive antibiotic therapy in monkeys and humans. In the present study, researchers analyzed 56 studies from the medical literature.
In 10 animal studies and 25 human studies (see table below), Lyme spirochetes were able to survive antibiotic therapy as shown by culture, tissue microscopy and xenodiagnosis (transfer of infection via tick bites).
Borrelia burgdorferi was detectable for 2-46 months after antibiotic therapy in rodents, dogs, monkeys, horses and humans.
“The presence of live spirochetes in symptomatic patients supports the role of ongoing infection in these patients,” said Lorraine Johnson. “When patients remain ill after antibiotic therapy, clinicians need to consider the possibility of persistent infection and the need for continued treatment.”
Dr. Stricker pointed to the implications for Lyme disease treatment raised by the study.
“This study is bad news for Lyme disease patients and their doctors,” he said. “We need to develop better antimicrobial treatments for these suffering patients, and we need to do it now.”
In the journal article’s acknowledgements, the authors wrote, “This article is dedicated to the memory of Pat Smith and Alan MacDonald.” Both individuals spent decades advancing understanding of the persistence of Lyme bacteria after antibiotic treatment, each contributing in their own distinct way.
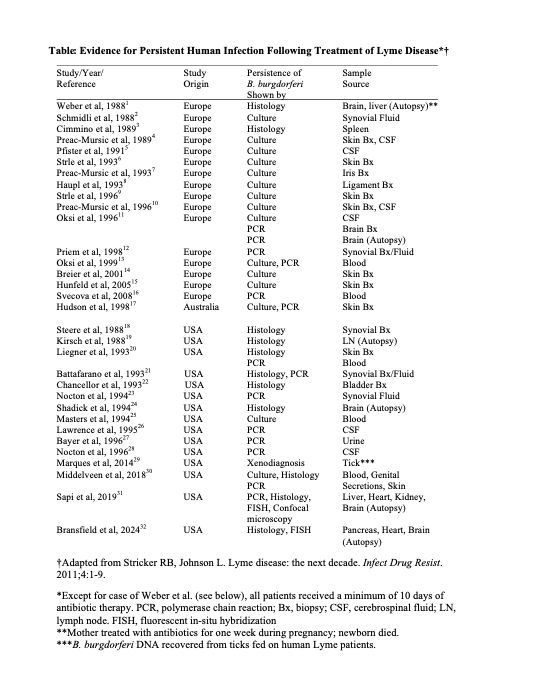 SOURCE: Union Square Medical Associates
SOURCE: Union Square Medical Associates


