C. Ben Beard, PhD1; James Occi, MA, MS2; Denise L. Bonilla, MS3; Andrea M. Egizi, PhD4; Dina M. Fonseca, PhD2; James W. Mertins, PhD3; Bryon P. Backenson, MS5; Waheed I. Bajwa, PhD6; Alexis M. Barbarin, PhD7; Matthew A. Bertone, PhD8; Justin Brown, DVM, PhD9; Neeta P. Connally, PhD10; Nancy D. Connell, PhD11; Rebecca J. Eisen, PhD1; Richard C. Falco, PhD5; Angela M. James, PhD3; Rayda K. Krell, PhD10; Kevin Lahmers, DVM, PhD12; Nicole Lewis, DVM13; Susan E. Little, DVM, PhD14; Michael Neault, DVM15; Adalberto A. Pérez de León, DVM, PhD16; Adam R. Randall, PhD17; Mark G. Ruder, DVM, PhD18; Meriam N. Saleh, PhD14; Brittany L. Schappach10; Betsy A. Schroeder, DVM19; Leslie L. Seraphin, DVM3; Morgan Wehtje, PhD3; Gary P. Wormser, MD20; Michael J. Yabsley, PhD21; William Halperin, MD, DrPH22 (View author affiliations)
Summary
What is already known about this topic?
Haemaphysalis longicornis is a tick indigenous to Asia, where it is an important vector of human and animal disease agents, which can result in human hemorrhagic fever and substantive reduction in dairy production.
What is added by this report?
During 2017–2018, H. longicornis has been detected in Arkansas, Connecticut, Maryland, New Jersey, New York, North Carolina, Pennsylvania, Virginia, and West Virginia on various species of domestic animals and wildlife, and from two humans.
What are the implications for public health practice?
The presence of H. longicornis in the United States represents a new and emerging disease threat. Characterization of the tick’s biology and ecology are needed, and surveillance efforts should include testing for potential indigenous and exotic pathogens.
Haemaphysalis longicornis is a tick indigenous to eastern Asia and an important vector of human and animal disease agents, resulting in such outcomes as human hemorrhagic fever and reduction of production in dairy cattle by 25%. H. longicornis was discovered on a sheep in New Jersey in August 2017 (1). This was the first detection in the United States outside of quarantine. In the spring of 2018, the tick was again detected at the index site, and later, in other counties in New Jersey, in seven other states in the eastern United States, and in Arkansas. The hosts included six species of domestic animals, six species of wildlife, and humans. To forestall adverse consequences in humans, pets, livestock, and wildlife, several critical actions are indicated, including expanded surveillance to determine the evolving distribution of H. longicornis, detection of pathogens that H. longicornis currently harbors, determination of the capacity of H. longicornis to serve as a vector for a range of potential pathogens, and evaluation of effective agents and methods for the control of H. longicornis.
H. longicornis is native to eastern China, Japan, the Russian Far East, and Korea. It is an introduced, and now established, exotic species in Australia, New Zealand, and several island nations in the western Pacific Region. Where this tick exists, it is an important vector of human and animal disease agents. In China and Japan, it transmits the severe fever with thrombocytopenia syndrome virus (SFTSV), which causes a human hemorrhagic fever (2), and Rickettsia japonica, which causes Japanese spotted fever (3). Studies in Asia identified ticks infected with various species of Anaplasma, Babesia, Borrelia, Ehrlichia, and Rickettsia, and all of these pathogen groups circulate zoonotically in the United States (4,5). In addition, parthenogenetic reproduction, a biologic characteristic of this species, allows a single introduced female tick to generate progeny without mating, thus resulting in massive host infestations. In some regions of New Zealand and Australia, this tick can reduce production in dairy cattle by 25% (6). Before 2017, H. longicornis ticks were intercepted at U.S. ports of entry at least 15 times on imported animals and materials (James W. Mertins, U.S. Department of Agriculture [USDA], personal communication).
The USDA Animal and Plant Inspection Service coordinated cooperative efforts through telephone conference calls with various local, state, and federal agricultural and public health agencies. Through these efforts, enhanced vector and animal surveillance were implemented to detect additional tick infestations. Suspect archival specimens that were available among previously collected ticks were also examined. Ticks were identified definitively by morphology at the USDA National Veterinary Services Laboratories or by DNA sequence analysis (molecular barcoding) at Rutgers University Center for Vector Biology, Monmouth County (New Jersey) Mosquito Control Division; College of Veterinary Medicine, University of Georgia; and Center for Veterinary Health Sciences, Oklahoma State University. By definition, a “report” is any new morphologic or molecular identification of H. longicornis ticks with a new county or host species from that county, identified from August 2017 through September 2018. Subsequent repeat collections are not reported here.
From August 2017 through September 2018, vector and animal surveillance efforts resulted in 53 reports of H. longicornis in the United States, including 38 (72%) from animal species (23 [61%] from domestic animals, 13 [34%] from wildlife, and two [5%] from humans), and 15 (28%) from environmental sampling of grass or other vegetation using cloth drags or flags* or carbon dioxide–baited tick traps.† With the exception of one report from Arkansas, the remaining reports of positively identified ticks are from eight eastern states: New Jersey (16; 30%), Virginia (15; 28%), West Virginia (11; 21%), New York (three; 6%), North Carolina (three; 6%), Pennsylvania (two; 4%), Connecticut (one; 2%), and Maryland (one; 2%) (Figure). Among the 546 counties or county equivalents in the nine states, ticks were reported from 45 (8%) counties (1.4% of all 3,109 U.S. counties and county equivalents) (Table 1). Excluding 15 reports of positive environmental sampling using flagging, dragging, or carbon dioxide traps, the remaining 38 reports reflect collection of ticks from infested host species (Table 2). Surveillance efforts did not include testing the ticks or hosts for pathogens. No cases of illness in humans or other species were reported. Concurrent reexamination of archived historical samples showed that invasion occurred years earlier. Most importantly, ticks collected from a deer in West Virginia in 2010 and a dog in New Jersey in 2013 were retrospectively identified as H. longicornis.
Discussion
Cooperative efforts among federal, state, and local experts from agricultural, public health, and academic institutions during the last year have documented that a tick indigenous to Asia is currently resident in several U.S. states. The public health and agricultural impacts of the multistate introduction and subsequent domestic establishment of H. longicornis are not known. At present, there is no evidence that H. longicornis has transmitted pathogens to humans, domestic animals, or wildlife in the United States. This species, however, is a potential vector of a number of important agents of human and animal diseases in the United States, including Rickettsia, Borrelia, Ehrlichia, Anaplasma, Theileria, and several important viral agents such as Heartland and Powassan viruses. Consequently, increased tick surveillance is warranted, using standardized animal and environmental sampling methods.
The findings in this report are subject to at least two limitations. First, the findings are limited by the variable surveillance methods used to identify the geographic and host distribution of H. longicornis. These methods included both passive and active surveillance. Conclusions about the geographic and host distribution might reflect the biases in the collection and submission of samples to states and USDA and the paucity of available information. Second, the data in this report reflect the collection of specimens that were positively identified by morphology or molecular barcoding. These represent sentinels that H. longicornis is present in different U.S. states and regions, and not a comprehensive assessment of the distribution of H. longicornis in the United States. The absence of positive samples from many states and counties might reflect the absence of infestation, absence of sampling, or failure to recover the tick. Even in states where H. longicornis has been found, the available data do not describe the actual extent or intensity of infestation.
The biology and ecology of H. longicornis as an exotic species in the United States should be characterized in terms of its vector competence (ability to transmit a pathogen) and vectorial capacity (feeding habits, host preference, climatic sensitivity, population density, and other factors that can affect the risk for pathogen transmission to humans) for tickborne pathogens known to be present in the United States (5). Surveillance for H. longicornis should include adequate sampling of companion animals, commercial animals, wildlife, and the environment. Where H. longicornis is detected, there should be testing for a range of indigenous and exotic viral, bacterial, and protozoan tickborne pathogens potentially transmitted by H. longicornis. Given the similarity between SFTSV and Heartland virus, a tickborne phlebovirus (https://www.cdc.gov/heartland-virus/index.html), further evaluation of the potential role of H. longicornis in transmission of this disease agent among animal reservoirs and possibly to humans is warranted. A broad range of interventions should be evaluated, including insecticide and acaricide sensitivity testing. Many state and federal agencies are developing and disseminating information for stakeholders, including development of hotlines, and some states are identifying ticks submitted by the public. The recently documented occurrence of H. longicornis in the United States presents an opportunity for collaboration among governmental, agricultural, public health agencies and partners in academic public health, veterinary sciences, and agricultural sciences to prevent diseases of potential national importance before onset in humans and other animal species.
Acknowledgments
Wes Watson, Andrew D. Haddow, Naomi Drexler, Gleeson Murphy, Harry Savage, Howard Ginsberg, Kim Cervantes, field and laboratory personnel.
References
- Rainey T, Occi JL, Robbins RG, Egizi A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J Med Entomol 2018;55:757–9. CrossRef PubMed
- Luo L-M, Zhao L, Wen H-L, et al. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis 2015;21:1770–6. CrossRef PubMed
- Mahara F. Japanese spotted fever: report of 31 cases and review of the literature. Emerg Infect Dis 1997;3:105–11. CrossRef PubMed
- Kang J-G, Ko S, Smith WB, Kim H-C, Lee I-Y, Chae J-S. Prevalence of Anaplasma, Bartonella and Borrelia species in Haemaphysalis longicornis collected from goats in North Korea. J Vet Sci 2016;17:207–16. CrossRef PubMed
- Rosenberg R, Lindsey NP, Fischer M, et al. Vital signs: trends in reported vectorborne disease cases—United States and territories, 2004–2016. MMWR Morb Mortal Wkly Rep 2018;67:496–501. CrossRef PubMed
- Heath A. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. N Z Vet J 2016;64:10–20. CrossRef PubMed
 FIGURE. Counties and county equivalents* where Haemaphysalis longicornis has been reported (N = 45) — United States, August 2017–September 2018
FIGURE. Counties and county equivalents* where Haemaphysalis longicornis has been reported (N = 45) — United States, August 2017–September 2018
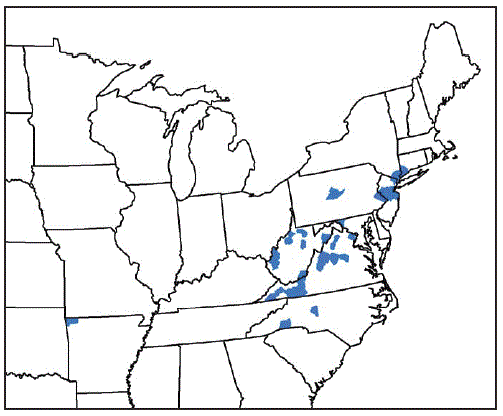 * Benton County, Arkansas; Fairfield County, Connecticut; Washington County, Maryland; Bergen, Hunterdon, Mercer, Middlesex, Monmouth, Somerset, and Union Counties, New Jersey; Davidson, Polk, and Rutherford Counties, North Carolina; Richmond, Rockland, and Westchester Counties, New York; Bucks and Centre Counties, Pennsylvania; Albemarle, Augusta, Carroll, Fairfax, Giles, Grayson, Louisa, Page, Pulaski, Rockbridge, Russell, Scott, Smyth, Staunton City, Warren, and Wythe Counties, Virginia; Cabell, Hardy, Lincoln, Mason, Marion, Monroe, Putnam, Ritchie, Taylor, Tyler, Upshur Counties, West Virginia.
* Benton County, Arkansas; Fairfield County, Connecticut; Washington County, Maryland; Bergen, Hunterdon, Mercer, Middlesex, Monmouth, Somerset, and Union Counties, New Jersey; Davidson, Polk, and Rutherford Counties, North Carolina; Richmond, Rockland, and Westchester Counties, New York; Bucks and Centre Counties, Pennsylvania; Albemarle, Augusta, Carroll, Fairfax, Giles, Grayson, Louisa, Page, Pulaski, Rockbridge, Russell, Scott, Smyth, Staunton City, Warren, and Wythe Counties, Virginia; Cabell, Hardy, Lincoln, Mason, Marion, Monroe, Putnam, Ritchie, Taylor, Tyler, Upshur Counties, West Virginia.
TABLE 1. Percentage of Haemaphysalis longicornis–infested counties or county equivalents in infested states — nine states, August 2017–September 2018
| State |
No. of counties* per state |
No. (%) of counties* with H. longicornis on host or in environment |
| Arkansas |
75 |
1 (1) |
| Connecticut |
8 |
1 (13) |
| Maryland |
24 |
1 (4) |
| New Jersey |
21 |
7 (33) |
| New York |
62 |
3 (5) |
| North Carolina |
100 |
3 (3) |
| Pennsylvania |
67 |
2 (3) |
| Virginia |
134 |
16 (12) |
| West Virginia |
55 |
11 (20) |
| Total |
546 |
45 (8) |
* Counties or county equivalents
TABLE 2. Distribution of Haemaphysalis longicornis, by host and species — nine states, August 2017–September 2018
| Host category, no. (% of total)/Species |
No. (% of host category) |
| Domestic animal, 23 (61) |
| Cat |
1 (4) |
| Cow |
4 (17) |
| Dog |
12 (52) |
| Goat |
2 (9) |
| Horse |
2 (9) |
| Sheep |
2 (9) |
| Total |
23 (100) |
| Wildlife, 13 (34) |
| Coyote |
1 (8) |
| White-tailed deer |
7 (54) |
| Gray fox |
1 (8) |
| Groundhog |
1 (8) |
| Virginia opossum |
2 (15) |
| Raccoon |
1 (8) |
| Total |
13 (100) |
| Human, 2 (5) |
2 (100) |
| Total |
38 (100) |
Beard CB, Occi J, Bonilla DL, et al. Multistate Infestation with the Exotic Disease–Vector Tick Haemaphysalis longicornis — United States, August 2017–September 2018. MMWR Morb Mortal Wkly Rep 2018;67:1310–1313. DOI: http://dx.doi.org/10.15585/mmwr.mm6747a3.
____________________
**Comment**
In the section discussing the species and the other pathogens it’s been known to transmit, Theileria was mentioned. Theileria is a malarial-like pathogen similar to Babesia:

 * Benton County, Arkansas; Fairfield County, Connecticut; Washington County, Maryland; Bergen, Hunterdon, Mercer, Middlesex, Monmouth, Somerset, and Union Counties, New Jersey; Davidson, Polk, and Rutherford Counties, North Carolina; Richmond, Rockland, and Westchester Counties, New York; Bucks and Centre Counties, Pennsylvania; Albemarle, Augusta, Carroll, Fairfax, Giles, Grayson, Louisa, Page, Pulaski, Rockbridge, Russell, Scott, Smyth, Staunton City, Warren, and Wythe Counties, Virginia; Cabell, Hardy, Lincoln, Mason, Marion, Monroe, Putnam, Ritchie, Taylor, Tyler, Upshur Counties, West Virginia.
* Benton County, Arkansas; Fairfield County, Connecticut; Washington County, Maryland; Bergen, Hunterdon, Mercer, Middlesex, Monmouth, Somerset, and Union Counties, New Jersey; Davidson, Polk, and Rutherford Counties, North Carolina; Richmond, Rockland, and Westchester Counties, New York; Bucks and Centre Counties, Pennsylvania; Albemarle, Augusta, Carroll, Fairfax, Giles, Grayson, Louisa, Page, Pulaski, Rockbridge, Russell, Scott, Smyth, Staunton City, Warren, and Wythe Counties, Virginia; Cabell, Hardy, Lincoln, Mason, Marion, Monroe, Putnam, Ritchie, Taylor, Tyler, Upshur Counties, West Virginia.