2018 Review of Previous Pathogen Transmission Time Studies in Deer Ticks
https://www.ncbi.nlm.nih.gov/pubmed/29398603
Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks.
Abstract
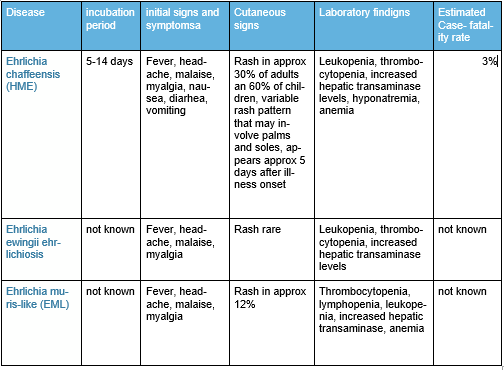
The blacklegged tick, Ixodes scapularis, is the primary vector to humans in the eastern United States of the deer tick virus lineage of Powassan virus (Powassan virus disease); the protozoan parasite Babesia microti (babesiosis); and multiple bacterial disease agents including Anaplasma phagocytophilum (anaplasmosis), Borrelia burgdorferi and Borrelia mayonii (Lyme disease), Borrelia miyamotoi (relapsing fever-like illness, named Borrelia miyamotoi disease), and Ehrlichia muris eauclairensis (a minor causative agent of ehrlichiosis).
With the notable exception of Powassan virus, which can be transmitted within minutes after attachment by an infected tick, there is no doubt that the risk of transmission of other I. scapularis-borne pathogens, including Lyme disease spirochetes, increases with the length of time (number of days) infected ticks are allowed to remain attached. This review summarizes data from experimental transmission studies to reinforce the important disease-prevention message that regular (at least daily) tick checks and prompt tick removal has strong potential to reduce the risk of transmission of I. scapularis-borne bacterial and parasitic pathogens from infected attached ticks.
The most likely scenario for human exposure to an I. scapularis-borne pathogen is the bite by a single infected tick. However, recent reviews have failed to make a clear distinction between data based on transmission studies where experimental hosts were fed upon by a single versus multiple infected ticks. A summary of data from experimental studies on transmission of Lyme disease spirochetes (Bo. burgdorferi and Bo. mayonii) by I. scapularis nymphs indicates that the probability of transmission resulting in host infection, at time points from 24 to 72 h after nymphal attachment, is higher when multiple infected ticks feed together as compared to feeding by a single infected tick.
In the specific context of risk for human infection, the most relevant experimental studies therefore are those where the probability of pathogen transmission at a given point in time after attachment was determined using a single infected tick. The minimum duration of attachment by single infected I. scapularis nymphs required for transmission to result in host infection is poorly defined for most pathogens, but experimental studies have shown that Powassan virus can be transmitted within 15 min of tick attachment and both A. phagocytophilum and Bo. miyamotoi within the first 24 h of attachment. There is no experimental evidence for transmission of Lyme disease spirochetes by single infected I. scapularis nymphs to result in host infection when ticks are attached for only 24 h (despite exposure of nearly 90 experimental rodent hosts across multiple studies) but the probability of transmission resulting in host infection appears to increase to approximately 10% by 48 h and reach 70% by 72 h for Bo. burgdorferi. Caveats to the results from experimental transmission studies, including specific circumstances (such as re-attachment of previously partially fed infected ticks) that may lead to more rapid transmission are discussed.
________________
**Comment**
There are a number of problematic issues with this study:
- This is a review of previous studies. There is nothing NEW here.
- It’s important to note that ticks typically carry more than just borrelia and transmission times have not taken this fact into account: https://madisonarealymesupportgroup.com/2017/05/01/co-infection-of-ticks-the-rule-rather-than-the-exception/ and https://www.lymedisease.org/lyme-basics/co-infections/about-co-infections/ Infection with more than one pathogen is associated with more severe illness.https://madisonarealymesupportgroup.com/2018/10/30/study-shows-lyme-msids-patients-infected-with-many-pathogens-and-explains-why-we-are-so-sick/ For the first time, Garg et al. show a 85% probability for multiple infections including not only tick-borne pathogens but also opportunistic microbes such as EBV and other viruses. This is a BIG DEAL. Finally, a study showing what we face as patients in the real world. They also never take into account nematodes (worms), mycoplasma, tularemia, and/or Bartonella. These are infections many if not most patients have to contend with. Some have been bioweaponized.
- They assume that the most likely scenario is for a person to be bitten by one tick. Assuming makes an ass out of u and me. When you take into account the latest information on the Asian tick, you quickly realize the probability of coming into contact with hundreds if not thousands of ticks at one time: https://madisonarealymesupportgroup.com/2018/09/12/three-surprising-things-i-learned-about-asian-longhorned-ticks-the-tick-guy-tom-mather/ While human infection has yet to be found in the U.S., this tick is responsible for plenty of misery in Asia: https://madisonarealymesupportgroup.com/2018/06/12/first-longhorned-tick-confirmed-in-arkansas/ It spreads SFTS (sever fever with thrombocytopenia syndrome), “an emerging hemorrhagic fever,” but the potential impact of this tick on tickborne illness is not yet known. In other parts of the world, it has been associated with several tickborne diseases, such as spotted fever rickettsioses, Anaplasma, Ehrlichia, and Borrelia, the causative agent of Lyme Disease.
- While they discuss the probability of multiple tick attachment, they never discuss the issue of partially fed ticks, where spirochetes would be in the salivary glands – leading to quicker transmission: http://iai.asm.org/content/61/6/2396.full.pdf Ticks can spontaneously detach – and the authors of this study found that they did so 15% of the time in mice. They also state that about a tenth of questing nymphs appear distended with partially fed sub-adult ticks being common.
- While the current review states, “There is no experimental evidence for transmission of Lyme disease spirochetes by single infected I. scapularis nymphs to result in host infection when ticks are attached for only 24 h (despite exposure of nearly 90 experimental rodent hosts across multiple studies), this study shows transmission can occur in under 16 hours: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4278789/
- https://madisonarealymesupportgroup.com/2017/04/14/transmission-time-for-lymemsids-infection/ Within this video, microbiologist Holly Ahern discusses the numerous problems with animal Bb transmission studies. Transmission Time: Only one study done on Mice. At 24 hours every tick had transmitted borrelia to the mice; however, animal studies have proven that transmission can occur in under 16 hours and it occurs frequently in under 24 hours. No human studies have been done and https://www.dovepress.com/lyme-borreliosis-a-review-of-data-on-transmission-time-after-tick-atta-peer-reviewed-article-IJGM no studies have determined the minimum time it takes for transmission. And, never forget the case of the little girl who couldn’t walk or talk after a tick bite attachment of 4-6 hours: https://madisonarealymesupportgroup.com/2016/12/07/igenex-presentation/
- They continue to blame Lyme/MSIDS on the black legged tick as the sole perp when experience and studies show there’s more potential transmitters at play: https://madisonarealymesupportgroup.com/2018/11/07/are-mosquitoes-transmitting-lyme-disease/, https://madisonarealymesupportgroup.com/2016/07/23/german-study-finds-borrelia-in-mosquitos/, https://madisonarealymesupportgroup.com/2019/01/17/remember-deer-keds-study-shows-bartonella-causing-deer-ked-dermatitis-in-humans/

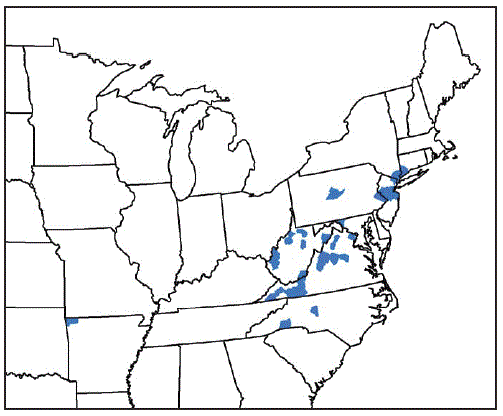 * Benton County, Arkansas; Fairfield County, Connecticut; Washington County, Maryland; Bergen, Hunterdon, Mercer, Middlesex, Monmouth, Somerset, and Union Counties, New Jersey; Davidson, Polk, and Rutherford Counties, North Carolina; Richmond, Rockland, and Westchester Counties, New York; Bucks and Centre Counties, Pennsylvania; Albemarle, Augusta, Carroll, Fairfax, Giles, Grayson, Louisa, Page, Pulaski, Rockbridge, Russell, Scott, Smyth, Staunton City, Warren, and Wythe Counties, Virginia; Cabell, Hardy, Lincoln, Mason, Marion, Monroe, Putnam, Ritchie, Taylor, Tyler, Upshur Counties, West Virginia.
* Benton County, Arkansas; Fairfield County, Connecticut; Washington County, Maryland; Bergen, Hunterdon, Mercer, Middlesex, Monmouth, Somerset, and Union Counties, New Jersey; Davidson, Polk, and Rutherford Counties, North Carolina; Richmond, Rockland, and Westchester Counties, New York; Bucks and Centre Counties, Pennsylvania; Albemarle, Augusta, Carroll, Fairfax, Giles, Grayson, Louisa, Page, Pulaski, Rockbridge, Russell, Scott, Smyth, Staunton City, Warren, and Wythe Counties, Virginia; Cabell, Hardy, Lincoln, Mason, Marion, Monroe, Putnam, Ritchie, Taylor, Tyler, Upshur Counties, West Virginia.