https://www.bostonglobe.com/magazine/2017/07/20/lyme-disease-turns-there-will-cake/vpP1zmsTVlakthjRApsHAJ/story.html By Mayeesha Galiba JULY 20, 2017
As to the percentage of LD in geographical areas, this is continually unfolding. Everyone is saying ticks are on the move – basically everywhere and have been found on every continent but Antarctica. Many states have not been reporting cases as well as don’t accept a case because it’s never been there before: https://madisonarealymesupportgroup.com/2016/09/24/arkansas-kids-denied-lyme-treatment/
Regarding percentage of ticks carrying Lyme Disease: https://www.lymedisease.org/lyme-basics/ticks/about-ticks/
Because tick studies have only been done in a relatively few places, in most of the US, tick infection rates are unknown. Even in places where ticks generally do not carry Lyme, there may be hotspots of infection depending on local conditions. The tick infection rate may also change from year to year, even in one location.
Percentage of folks seeing a rash is far lower and ranges from 27%-80%: 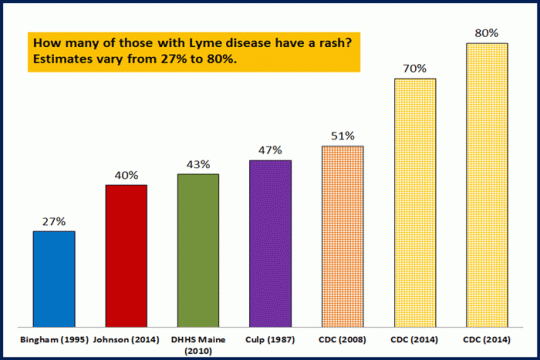 https://www.lymedisease.org/lymepolicywonk-how-many-of-those-with-lyme-disease-have-the-rash-estimates-range-from-27-80-2/ Hardly anyone I know sees an EM rash.
https://www.lymedisease.org/lymepolicywonk-how-many-of-those-with-lyme-disease-have-the-rash-estimates-range-from-27-80-2/ Hardly anyone I know sees an EM rash.
 https://www.lymedisease.org/lymepolicywonk-how-many-of-those-with-lyme-disease-have-the-rash-estimates-range-from-27-80-2/ Hardly anyone I know sees an EM rash.
https://www.lymedisease.org/lymepolicywonk-how-many-of-those-with-lyme-disease-have-the-rash-estimates-range-from-27-80-2/ Hardly anyone I know sees an EM rash.As to the Lyme vaccine being pulled from the market due to lack of demand:
Did you know that the LYMERIX vaccine caused 640 emergency room visits, 34 life threatening reactions, 77 hospitalizations, 198 disabilities, and 6 deaths? In a vile cesspool of conflicts of interest are university patent holders, drug companies, and the FDA itself as another patent holder. It generated 40 million dollars before it was yanked. (2008, Drymon)
http://www.yourlawyer.com/topics/overview/lymerix One doctor stated that 21 patients developed severe arthritis after receiving the LYMERIX vaccine.
http://www.yourlawyer.com/topics/overview/lymerix One doctor stated that 21 patients developed severe arthritis after receiving the LYMERIX vaccine.
http://www.lymediseaseassociation.org/index.php/about-lyme/controversy/vaccine/261-lymerix-meeting “Given that Dr. Marks lead the clinical trials for Lymerix’s competitor, the OspA vaccine produced and then abandoned by Aventis Pasteur, his conclusions mean a lot. “In my opinion,” he told FDA officials, “there is sufficient evidence that Lymerix is causally related to severe rheumatologic, neurologic, autoimmune, and other adverse events in some individuals. This evidence is such as to warrant a significantly heightened degree of warnings and possible limitations or removal from marketing of Lymerix.”
https://madisonarealymesupportgroup.com/2017/01/26/lyme-vaccine-to-be-tested-on-humans/ The biological mechanism hypothesis was that the outer surface protein A (OspA), which was the antigenic component of the LYMErix vaccine, induced autoimmunity in genetically susceptible individuals, including high levels of autoantibody to OspA in their synovial fluid.
Dr. Stricker states:
Another Lyme OspA Vaccine Whitewash https://www.ncbi.nlm.nih.gov/pubmed/28141584/#comments
The meta-analysis by Zhao and colleagues comes to the conclusion that “the OspA vaccine against Lyme disease is safe and its immunogenicity and efficacy have been verified.” The authors arrive at this sunny conclusion by excluding 99.6% of published articles that demonstrate potential problems with the OspA vaccine. Furthermore, the authors ignore peer-reviewed studies, FDA regulatory meetings and legal proceedings that point to major problems with OspA vaccine safety (1-3). This whitewash bodes ill for future Lyme vaccine candidates because it fosters disregard for vaccine safety among Lyme vaccine manufacturers and mistrust among potential Lyme vaccinees.
Another Lyme OspA Vaccine Whitewash https://www.ncbi.nlm.nih.gov/pubmed/28141584/#comments
The meta-analysis by Zhao and colleagues comes to the conclusion that “the OspA vaccine against Lyme disease is safe and its immunogenicity and efficacy have been verified.” The authors arrive at this sunny conclusion by excluding 99.6% of published articles that demonstrate potential problems with the OspA vaccine. Furthermore, the authors ignore peer-reviewed studies, FDA regulatory meetings and legal proceedings that point to major problems with OspA vaccine safety (1-3). This whitewash bodes ill for future Lyme vaccine candidates because it fosters disregard for vaccine safety among Lyme vaccine manufacturers and mistrust among potential Lyme vaccinees.

